WHAT IS ATOMS?
Atoms are the smallest part of an element that still has its properties. Moreover, it is also the necessary ingredient for an object to be consider matter. Every atom is consist of a nucleus with electrons orbit around it. The nucleus embody by protons and neutrons. Protons are positive charge, neutrons possess no charge and electrons are negative charge. Everything in your daily life is make of atoms.
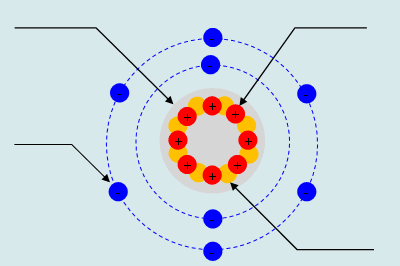
Nucleus
Protons
Neurons
Electrons




SIMPLE MOLECULES
Molecules are atoms that are chemically combined. However, there are different types of molecules. Elements have only one type of atom bonding. Meanwhile, two or more types of atoms combined and shared electrons is called compound. A closely-related example that is well-known by everyone is water. Both fresh and salt water are the outcome of two hydrogen atoms bonding with one oxygen atom.

O
H
H

EXTENDED STRUCTURE
Extended structure is in other words, a chain of molecules synthetically intergrate. Polymers are types of extended structure. It is a large molecule that is made up of repeating monomers. One familiar illustration is polypropylene. Its building blocks are C3H6. To succesfully create this synthetic imtermediate , repetition of C3H6 are needed. As we can all perceive, polypropylene are everywhere. Stationeries are especially made out of polypropylene such as binders, pens, paper clips and even office chairs. Therefore, this extended structure are no doubt very familiar with us.

STATE OF MATTER
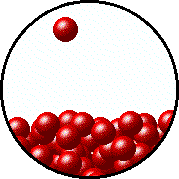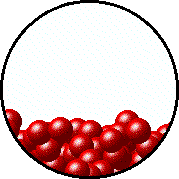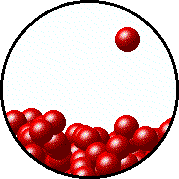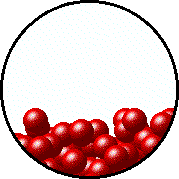



A distinctive form that matter could transform to is called state of matter. Involved for the three fundamental states, they all have distinguished particles motion. Solid form have fixed shape and atoms only vibrates in place. Gas particles flow freely with no boundaries and volume. Water's properties lied between solid and gas form. Their particles don't move without restriction and also don't stay still, but able to slide through each other in a not definite shape. The fourth one, but nonessential, is plasma. Inside this state of matter, nucleuses and electrons are segregrate and these particles moved at its pleasure. However, we will only be focus in solid, liquid and gas.

In spite of developing conversions between different state of matter, addition of thermal energy is required. An escalation in heat would interrupt and disconnect the matter's current structure, lead to another state. In other words, the particles would gain more energy which result as violent movements and disjoint the current state's form. And vice versa, the reduction in temperature would link the atoms back together. For instance, H2O can be presented in many stages in our daily life. Its solid state is ice cubes, which is the result of frozen water. H2O's most well-known form is normal water in liquid form was then created through melting. Boiling and vaporization, are expressions for the change from liquid to gas form of H2O. The representative for gas in H2O form is water vapor. Furtheremore, there are also method such as sublimation and deposition to transit from solid to gas or reverse.